





Melatonin is a natural compound, specifically an indoleamine, produced by and found in different organisms including bacteria and eukaryotes.[4] It was discovered by Aaron B. Lerner and colleagues in 1958 as a substance of the pineal gland from cow that could induce skin lightening in common frogs. It was subsequently discovered as a hormone released in the brain at night which controls the sleep–wake cycle in vertebrates.[2][5]
In vertebrates, melatonin is involved in synchronizing circadian rhythms, including sleep–wake timing and blood pressure regulation, and in control of seasonal rhythmicity including reproduction, fattening, moulting and hibernation.[6] Many of its effects are through activation of the melatonin receptors, while others are due to its role as an antioxidant.[7][8][9] Its primary function is to defend against oxidative stress in plants[10] and bacteria. Mitochondria are the main cell organelles that produce the antioxidant melatonin,[11] which indicates that melatonin is an "ancient molecule" that primarily provided the earliest cells protection from the destructive actions of oxygen.[12][13]
In addition to its role as a natural hormone and antioxidant, melatonin is used as a dietary supplement and medication in the treatment of sleep disorders such as insomnia and circadian rhythm sleep disorders.
In humans, melatonin is a full agonist of melatonin receptor 1 (picomolar binding affinity) and melatonin receptor 2 (nanomolar binding affinity), both of which belong to the class of G-protein coupled receptors (GPCRs).[14][15] Melatonin receptors 1 and 2 are both Gi/o-coupled GPCRs, although melatonin receptor 1 is also Gq-coupled.[14] Melatonin also acts as a high-capacity free radical scavenger within mitochondria which also promotes the expression of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase via signal transduction through melatonin receptors.[16][14][17][18][19][20]
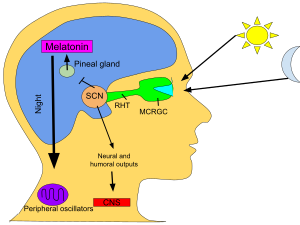
In animals, melatonin plays an important role in the regulation of sleep–wake cycles.[21] Human infants' melatonin levels become regular in about the third month after birth, with the highest levels measured between midnight and 8:00 am.[22] Human melatonin production decreases as a person ages.[23] Also, as children become teenagers, the nightly schedule of melatonin release is delayed, leading to later sleeping and waking times.[24]
Melatonin was first reported as a potent antioxidant and free radical scavenger in 1993.[25] In vitro, melatonin acts as a direct scavenger of oxygen radicals including OH•, O2−•, and the reactive nitrogen species NO•.[26][27] In plants, melatonin works with other antioxidants to improve the overall effectiveness of each antioxidant.[27] Melatonin has been proven to be twice as active as vitamin E, believed to be the most effective lipophilic antioxidant.[28] Via signal transduction through melatonin receptors, melatonin promotes the expression of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase.[16][14]
Melatonin occurs at high concentrations within mitochondrial fluid which greatly exceed the plasma concentration of melatonin.[17][18][19] Due to its capacity for free radical scavenging, indirect effects on the expression of antioxidant enzymes, and its significant concentrations within mitochondria, a number of authors have indicated that melatonin has an important physiological function as a mitochondrial antioxidant.[16][17][18][19][20]
The melatonin metabolites produced via the reaction of melatonin with reactive oxygen species or reactive nitrogen species also react with and reduce free radicals.[14][20] Melatonin metabolites generated from redox reactions include cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK).[14][20]
While it is known that melatonin interacts with the immune system,[29][30] the details of those interactions are unclear. An anti-inflammatory effect seems to be the most relevant[citation needed]. There have been few trials designed to judge the effectiveness of melatonin in disease treatment. Most existing data are based on small, incomplete trials. Any positive immunological effect is thought to be the result of melatonin acting on high-affinity receptors (MT1 and MT2) expressed in immunocompetent cells. In preclinical studies, melatonin may enhance cytokine production and stimulate T cell expansion,[31] and by doing this, counteract acquired immunodeficiences.[32]
A possible mechanism by which melatonin may regulate weight gain is through its inhibitory effect on leptin.[33] Leptin acts as a long-term indicator of energy status in the human body.[34] By suppressing leptin's actions outside of waking hours, melatonin may help restore leptin sensitivity during the daytime by alleviating leptin resistance.[33][35]

In animals, biosynthesis of melatonin occurs through hydroxylation, decarboxylation, acetylation and a methylation starting with L-tryptophan.[36] L-tryptophan is produced in the shikimate pathway from chorismate or is acquired from protein catabolism. First L-tryptophan is hydroxylated on the indole ring by tryptophan hydroxylase to produce 5-hydroxytryptophan. This intermediate (5-HTP) is decarboxylated by pyridoxal phosphate and 5-hydroxytryptophan decarboxylase to produce serotonin.
Serotonin is itself an important neurotransmitter, but is also converted into N-acetylserotonin by serotonin N-acetyltransferase with acetyl-CoA.[37] Hydroxyindole O-methyltransferase and S-adenosyl methionine convert N-acetylserotonin into melatonin through methylation of the hydroxyl group.[37]
In bacteria, protists, fungi, and plants, melatonin is synthesized indirectly with tryptophan as an intermediate product of the shikimate pathway. In these cells, synthesis starts with D-erythrose 4-phosphate and phosphoenolpyruvate, and in photosynthetic cells with carbon dioxide. The rest of the synthesizing reactions are similar, but with slight variations in the last two enzymes.[38][39]
It has been hypothesized that melatonin is made in the mitochondria and chloroplasts.[40]

In order to hydroxylate L-tryptophan, the cofactor tetrahydrobiopterin (THB) must first react with oxygen and the active site iron of tryptophan hydroxylase. This mechanism is not well understood, but two mechanisms have been proposed:
1. A slow transfer of one electron from the THB to O2 could produce a superoxide which could recombine with the THB radical to give 4a-peroxypterin. 4a-peroxypterin could then react with the active site iron (II) to form an iron-peroxypterin intermediate or directly transfer an oxygen atom to the iron.
2. O2 could react with the active site iron (II) first, producing iron (III) superoxide which could then react with the THB to form an iron-peroxypterin intermediate.
Iron (IV) oxide from the iron-peroxypterin intermediate is selectively attacked by a double bond to give a carbocation at the C5 position of the indole ring. A 1.2-shift of the hydrogen and then a loss of one of the two hydrogen atoms on C5 reestablishes aromaticity to furnish 5-hydroxy-L-tryptophan.[41]
A decarboxylase with cofactor pyridoxal phosphate (PLP) removes CO2 from 5-hydroxy-L-tryptophan to produce 5-hydroxytryptamine.[42] PLP forms an imine with the amino acid derivative. The amine on the pyridine is protonated and acts as an electron sink, enabling the breaking of the C-C bond and releasing CO2. Protonation of the amine from tryptophan restores the aromaticity of the pyridine ring and then imine is hydrolyzed to produce 5-hydroxytryptamine and PLP.[43]
It has been proposed that histidine residue His122 of serotonin N-acetyl transferase is the catalytic residue that deprotonates the primary amine of 5-hydroxytryptamine, which allows the lone pair on the amine to attack acetyl-CoA, forming a tetrahedral intermediate. The thiol from coenzyme A serves as a good leaving group when attacked by a general base to give N-acetylserotonin.[44]
N-Acetylserotonin is methylated at the hydroxyl position by S-adenosyl methionine (SAM) to produce S-adenosyl homocysteine (SAH) and melatonin.[43][45]
In vertebrates, melatonin secretion is regulated by activation of the beta-1 adrenergic receptor by norepinephrine.[46] Norepinephrine elevates the intracellular cAMP concentration via beta-adrenergic receptors and activates the cAMP-dependent protein kinase A (PKA). PKA phosphorylates the penultimate enzyme, the arylalkylamine N-acetyltransferase (AANAT). On exposure to (day)light, noradrenergic stimulation stops and the protein is immediately destroyed by proteasomal proteolysis.[47] Production of melatonin is again started in the evening at the point called the dim-light melatonin onset.
Blue light, principally around 460–480 nm, suppresses melatonin biosynthesis,[48] proportional to the light intensity and length of exposure. Until recent history, humans in temperate climates were exposed to few hours of (blue) daylight in the winter; their fires gave predominantly yellow light.[49] The incandescent light bulb widely used in the 20th century produced relatively little blue light.[50] Light containing only wavelengths greater than 530 nm does not suppress melatonin in bright-light conditions.[51] Wearing glasses that block blue light in the hours before bedtime may decrease melatonin loss.[52] Use of blue-blocking goggles the last hours before bedtime has also been advised for people who need to adjust to an earlier bedtime, as melatonin promotes sleepiness.[53]
Melatonin has an elimination half-life of 20 to 50 minutes.[1][2][3] In humans, melatonin is mainly metabolized to 6-hydroxymelatonin, which is conjugated with sulfate to be excreted as a waste product in urine.[54]
For research as well as clinical purposes, melatonin concentration in humans can be measured either from the saliva or blood plasma.[55]
Main article: Melatonin as a medication and supplement
Melatonin is used as a prescription medication and over-the-counter dietary supplement in the treatment of sleep disorders such as insomnia and circadian rhythm sleep disorders like delayed sleep phase disorder, jet lag disorder, and shift work disorder.[56] Besides melatonin, certain synthetic melatonin receptor agonists like ramelteon, tasimelteon, and agomelatine are also used in medicine.[57][58] Emerging evidence suggests melatonin may mitigate self-harm risks in adolescents. [59]
A study by the Journal of the American Medical Association published in April 2023 found that only 12% of the 30 preparations analyzed contained quantities of melatonin that were within ±10% of the declared dosage. Some supplements contained up to 347% of the declared quantity. Melatonin is an active pharmaceutical ingredient in Europe, while the U.S. in 2022 considered the substance for inclusion in pharmacy compounding. A previous study from 2022 also concluded that consuming unregulated melatonin products 'as directed' could expose children to between 40 and 130 times higher quantities of melatonin than indicated.[60]
| melatonin gummies sleep cbd supplements health product products people effects time supplement gummy children research ingredients doctor body dosage milligrams team foundation dose minutes night adults editorial sugar side quality insomnia hormone aid tablets blood food hours disorder content |
lag melatonin gummies melatonin supplements side effects sleep gummies cbd gummies tni editorial team read time pros cons jet lag sleep disorders melatonin dosage sleep problems medical advice nordic naturals sleep quality valerian root sleep aid sugar melatonin gummies melatonin gummy drug administration melatonin supplement good night view source national center medical professional user reviews united states sleep aids sleep products lemon balm circadian rhythm many people active ingredients sleep-wake phase disorder daily habits sleep quiz |
sleep improvement journey deerforia |




Melatonin is primarily used for sleep regulation and is not typically associated with improving mood or happiness.
The use of sleeping pills in adolescents should be discussed with a healthcare provider for safety and proper dosing.
4 to 5 hours of sleep is below the recommended amount for most adults and may lead to sleep deprivation.
It's generally safe to take melatonin after drinking alcohol, but moderation is essential as alcohol can disrupt sleep.
The duration of melatonin use should be discussed with a healthcare provider; it's not typically recommended for long-term daily use.
There are other sleep aids and lifestyle changes that may be considered as alternatives to melatonin. Consult a healthcare provider for options.
Taking melatonin at 3 am may help if you plan to go back to sleep; consult a healthcare provider for personalized advice.
Yes, 15 mg of melatonin is a very high dose and should only be used under strict medical supervision.
Maintaining a consistent sleep schedule, creating a comfortable sleep environment, and practicing good sleep hygiene can help you achieve 8 hours of sleep.
It's generally safe to take melatonin after drinking alcohol, but moderation is essential as alcohol can disrupt sleep.
Common side effects include dizziness, headaches, and grogginess. Rare side effects may include nightmares or increased anxiety.